12 Types of Filtration Techniques For Different Industrial
Filtration is a technique used to separate solid particles from a fluid (liquid or gas) by passing the fluid through a medium that retains the solid particles. Depending on the nature of the fluid and the solid, the size of the particles, the purpose of the filtration, and other factors, different filtration techniques are employed. Here we list 12 kinds of main types of filtration techniques commonly used in various industries, hope those can be helpful for you know more details about filtration.
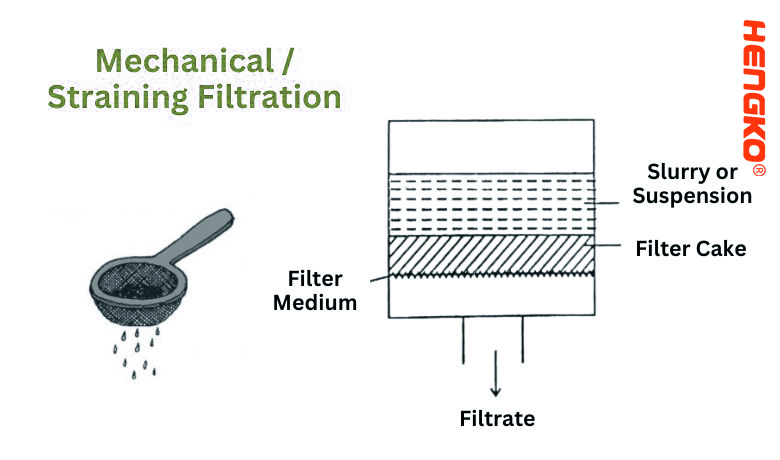
1. Mechanical / Straining Filtration:
Mechanical/Straining Filtration is one of the simplest and most straightforward filtration methods. At its core, it involves passing a fluid (either liquid or gas) through a barrier or medium that stops or captures particles larger than a certain size, while allowing the fluid to pass through.
1.) Key Characteristics:
* Filter Medium: The filter medium typically has small openings or pores whose size determines which particles will be trapped and which will flow through. The medium can be made from various materials, including fabrics, metals, or plastics.
* Particle Size: Mechanical filtration is primarily concerned with particle size. If a particle is larger than the pore size of the filter medium, it gets trapped or strained out.
* Flow Pattern: In most mechanical filtration setups, the fluid flows perpendicularly to the filter medium.
2.) Common Applications:
* Household Water Filters: Basic water filters that remove sediments and larger contaminants rely on mechanical filtration.
* Coffee Brewing: A coffee filter acts as a mechanical filter, allowing the liquid coffee to pass through while retaining the solid coffee grounds.
* Swimming Pools: Pool filters often use a mesh or screen to trap larger debris like leaves and insects.
* Industrial Processes: Many manufacturing processes require the removal of larger particles from liquids, and mechanical filters are frequently employed.
* Air Filters in HVAC Systems: These filters trap larger airborne particles like dust, pollen, and some microbes.
3.) Advantages:
* Simplicity: Mechanical filtration is easy to understand, implement, and maintain.
* Versatility: By varying the material and pore size of the filter medium, mechanical filtration can be adapted for a wide range of applications.
* Cost-effective: Due to its simplicity, the initial and maintenance costs are often lower than for more complex filtration systems.
4.) Limitations:
* Clogging: Over time, as more and more particles are trapped, the filter can become clogged, reducing its efficiency and requiring cleaning or replacement.
* Limited to Larger Particles: Mechanical filtration is not effective for removing very small particles, dissolved substances, or certain microorganisms.
* Maintenance: Regular checking and replacement or cleaning of the filter medium is essential to maintain efficiency.
In conclusion, mechanical or straining filtration is a foundational method of separation based on particle size. While it may not be suitable for applications requiring the removal of very small particles or dissolved substances, it's a reliable and efficient method for many everyday and industrial applications.
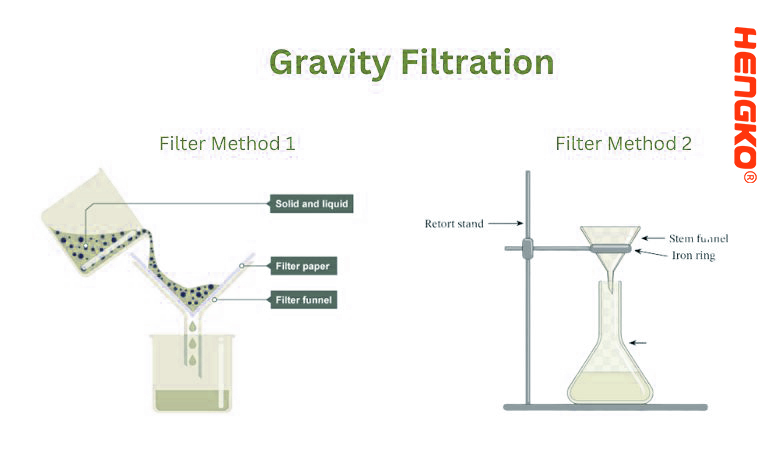
2. Gravity Filtration:
Gravity Filtration is a technique primarily used in the laboratory to separate a solid from a liquid using the force of gravity. This method is suitable when the solid is insoluble in the liquid or when you want to remove impurities from a liquid.
1.) Process:
* A circular filter paper, usually made of cellulose, is folded and placed in a funnel.
* The mixture of solid and liquid is poured onto the filter paper.
* Under the influence of gravity, the liquid passes through the pores of the filter paper and gets collected below, while the solid remains on the paper.
2.) Key Characteristics:
* Filter Medium: Typically, a qualitative filter paper is used. The choice of filter paper depends on the size of the particles to be separated and the rate of filtration required.
* Equipment: A simple glass or plastic funnel is often used. The funnel is placed on a ring stand above a flask or beaker to collect the filtrate
(the liquid that has passed through the filter).
* No External Pressure: Unlike vacuum filtration, where an external pressure difference speeds up the process, gravity filtration relies solely on gravitational force. This means it's generally slower than other methods like vacuum or centrifugal filtration.
3) Common Applications:
* Laboratory Separations:
Gravity filtration is a common technique in chemistry laboratories for simple separations or for removing impurities from solutions.
* Making Tea: The process of making tea using a tea bag is essentially a form of gravity filtration,
where the liquid tea passes through the bag (acting as the filter medium), leaving behind the solid tea leaves.
4.) Advantages:
* Simplicity: It's a straightforward method that requires minimal equipment, making it accessible and easy to understand.
* No Need for Electricity: Since it doesn't rely on external pressure or machinery, gravity filtration can be done without any power sources.
* Safety: With no pressure build-up, there's a reduced risk of accidents compared to pressurized systems.
5.) Limitations:
* Speed: Gravity filtration can be slow, especially when filtering mixtures with fine particles or high solid content.
* Not Ideal for Very Fine Particles: Extremely small particles may pass through the filter paper or cause it to clog quickly.
* Limited Capacity: Due to its reliance on simple funnels and filter papers, it's not suitable for large-scale industrial processes.
In summary, gravity filtration is a simple and straightforward method of separating solids from liquids. While it may not be the fastest or most efficient method for all scenarios, its ease of use and minimal equipment requirements make it a staple in many laboratory settings.
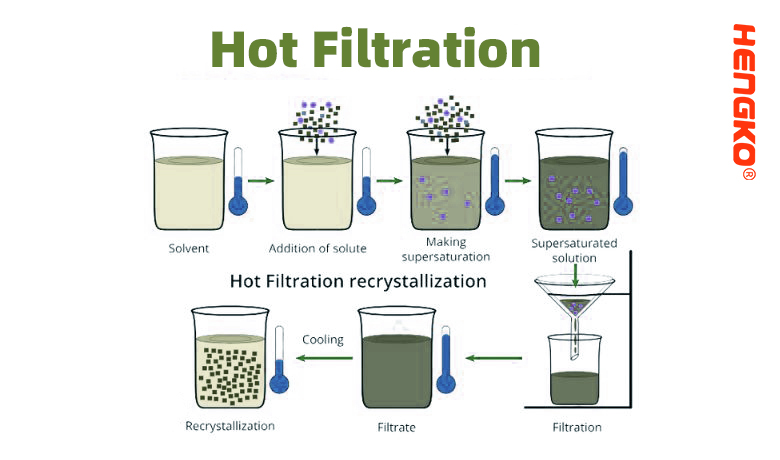
3. Hot Filtration
Hot filtration is a laboratory technique utilized to separate insoluble impurities from a hot saturated solution before it cools and crystallizes. The main purpose is to remove the impurities that might be present, ensuring that they don't get incorporated into the desired crystals upon cooling.
1.) Procedure:
* Heating: The solution containing the desired solute and impurities is first heated to dissolve the solute completely.
* Setting Up the Apparatus: A filter funnel, preferably one made of glass, is placed on a flask or beaker. A piece of filter paper is placed inside the funnel. To prevent premature crystallization of the solute during filtration, the funnel is often heated using a steam bath or a heating mantle.
* Transfer: The hot solution is poured into the funnel, allowing the liquid portion (filtrate) to pass through the filter paper and collect in the flask or beaker below.
* Trapping Impurities: Insoluble impurities are left behind on the filter paper.
2.) Key Points:
* Maintain Temperature: It's crucial to keep everything hot during the process.
Any drop in temperature can result in the desired solute crystallizing on the filter paper along with the impurities.
* Fluted Filter Paper: Often, the filter paper is fluted or folded in a specific manner to increase its surface area, promoting faster filtration.
* Steam Bath or Hot Water Bath: This is commonly used to keep the funnel and the solution warm, reducing the risk of crystallization.
3.) Advantages:
* Efficiency: Allows for the removal of impurities from a solution before crystallization, ensuring pure crystals.
* Clarity: Helps in obtaining a clear filtrate devoid of insoluble contaminants.
4.) Limitations:
* Heat Stability: Not all compounds are stable at elevated temperatures, which might limit the use of hot filtration for some sensitive compounds.
* Safety Concerns: Handling hot solutions increases the risk of burns and requires extra precautions.
* Equipment Sensitivity: Special attention must be given to the glassware as rapid temperature changes can cause it to crack.
In summary, hot filtration is a technique specifically designed for the separation of impurities from a hot solution, ensuring that the resulting crystals upon cooling are as pure as possible. Proper techniques and safety precautions are essential for effective and safe results.
4. Cold filtration
Cold Filtration is a method employed mainly in the laboratory to separate or purify substances. As the name suggests, cold filtration involves cooling the solution, typically to promote the separation of unwanted materials.
1. Procedure:
* Cooling the Solution: The solution is cooled, often in an ice bath or a refrigerator. This cooling process will cause unwanted substances (often impurities) that are less soluble at low temperatures to crystallize out of the solution.
* Setting Up the Apparatus: Just like in other filtration techniques, a filter funnel is placed on top of a receiving vessel (like a flask or beaker). A filter paper is positioned inside the funnel.
* Filtration: The cold solution is poured into the funnel. The solid impurities, which have crystallized due to the reduced temperature, are trapped on the filter paper. The purified solution, known as the filtrate, collects in the vessel below.
Key Points:
* Purpose: Cold filtration is mainly used to remove impurities or unwanted substances that become insoluble or less soluble at reduced temperatures.
* Precipitation: The technique can be used in tandem with precipitation reactions, where a precipitate forms upon cooling.
* Solubility: Cold filtration takes advantage of the reduced solubility of some compounds at lower temperatures.

Advantages:
* Purity: It provides a way to enhance the purity of a solution by removing unwanted components that crystallize out upon cooling.
* Selective Separation: Since only certain compounds will precipitate or crystallize at specific temperatures, cold filtration can be used for selective separations.
Limitations:
* Incomplete Separation: Not all impurities might crystallize or precipitate upon cooling, so some contaminants could still remain in the filtrate.
* Risk of Losing Desired Compound: If the compound of interest also has reduced solubility at lower temperatures, it might crystallize out along with the impurities.
* Time-Consuming: Depending on the substance, reaching the desired low temperature and allowing impurities to crystallize can be time-consuming.
In summary, cold filtration is a specialized technique that makes use of temperature changes to achieve separation. The method is especially useful when certain impurities or components are known to crystallize or precipitate at lower temperatures, allowing for their separation from the main solution. As with all techniques, understanding the properties of the substances involved is crucial for effective results.
5. Vacuum Filtration:
Vacuum filtration is a fast filtration technique used to separate solids from liquids. By applying a vacuum to the system, the liquid is drawn through the filter, leaving the solid residues behind. It is particularly useful for separating large quantities of residue or when the filtrate is a viscous or slow-moving liquid.
1.) Procedure:
* Setting Up the Apparatus: A Büchner funnel (or a similar funnel designed for vacuum filtration) is positioned on top of a flask, often called a filter flask or Büchner flask. The flask is connected to a vacuum source. A piece of filter paper or a sintered glass disc is placed inside the funnel to act as the filtering medium.
* Applying Vacuum: The vacuum source is turned on, reducing the pressure inside the flask.
* Filtration: The liquid mixture is poured onto the filter. The reduced pressure in the flask draws the liquid (filtrate) through the filter medium, leaving the solid particles (residue) on top.
2.) Key Points:
* Speed: The application of a vacuum significantly speeds up the filtration process compared to gravity-driven filtration.
* Seal: A good seal between the funnel and flask is crucial to maintain the vacuum. Often, this seal is achieved using a rubber or silicone bung.
* Safety: When using glass apparatus under vacuum, there's a risk of implosion. It's essential to ensure that all glassware is free of cracks or
defects and to shield the setup when possible.
3.) Advantages:
* Efficiency: Vacuum filtration is much faster than simple gravity filtration.
* Versatility: It can be used with a wide range of solutions and suspensions, including those that are highly viscous or have a large amount of solid residue.
* Scalability: Suitable for both small-scale laboratory procedures and larger industrial processes.
4.) Limitations:
* Equipment Requirement: Requires additional equipment, including a vacuum source and specialized funnels.
* Risk of Clogging: If the solid particles are very fine, they might clog the filter medium, slowing down or halting the filtration process.
* Safety Concerns: The use of a vacuum with glassware introduces risks of implosion, necessitating proper safety precautions.
In summary, vacuum filtration is a powerful and efficient method for separating solids from liquids, especially in scenarios where rapid filtration is desirable or when dealing with solutions that are slow to filter under the force of gravity alone. Proper setup, equipment checks, and safety precautions are essential to ensure successful and safe results.
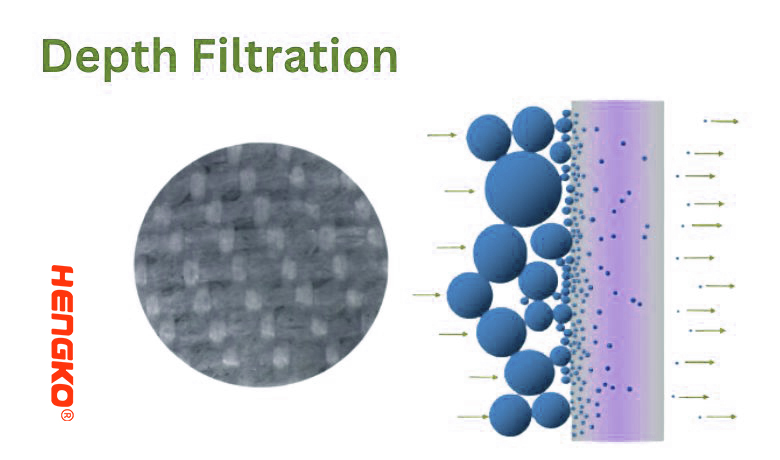
6. Depth Filtration:
Depth filtration is a filtration method in which particles are captured within the thickness (or "depth") of the filter medium, rather than just on the surface. The filter medium in depth filtration is typically a thick, porous material that traps particles throughout its structure.
1.) Mechanism:
* Direct Interception: Particles are directly captured by the filter medium as they come into contact with it.
* Adsorption: Particles adhere to the filter medium due to van der Waals forces and other attractive interactions.
* Diffusion: Small particles move erratically due to Brownian motion and eventually get trapped within the filter medium.
2.) Materials:
Common materials used in depth filtration include:
* Cellulose
* Diatomaceous earth
* Perlite
* Polymeric resins
3.) Procedure:
* Preparation: The depth filter is set up in a manner that forces the liquid or gas to pass through its entire thickness.
* Filtration: As the fluid flows through the filter medium, particles are trapped throughout the depth of the filter, not just on the surface.
* Replacement / Cleaning: Once the filter medium becomes saturated or the flow rate drops significantly, it needs to be replaced or cleaned.
4.) Key Points:
* Versatility: Depth filters can be used to filter a wide range of particle sizes, from relatively large particles to very fine ones.
* Gradient Structure: Some depth filters have a gradient structure, meaning the pore size varies from the inlet to the outlet side. This design allows for more efficient particle capture as larger particles are trapped near the inlet while finer particles are captured deeper within the filter.
5.) Advantages:
* High Dirt Holding Capacity: Depth filters can hold a significant amount of particles due to the volume of the filter material.
* Tolerance to Varied Particle Sizes: They can handle fluids with a wide range of particle sizes.
* Reduced Surface Clogging: Since particles are trapped throughout the filter medium, depth filters tend to experience less surface clogging compared to surface filters.
6.) Limitations:
* Replacement Frequency: Depending on the nature of the fluid and the amount of particulate, depth filters can become saturated and need replacement.
* Not Always Regenerable: Some depth filters, especially those made of fibrous materials, may not be easily cleaned and regenerated.
* Pressure Drop: The thick nature of depth filters can lead to a higher pressure drop across the filter, especially as it begins to fill with particles.
In summary, depth filtration is a method used to capture particles within the structure of a filter medium, rather than just on the surface. This method is especially useful for fluids with a wide range of particle sizes or when a high dirt holding capacity is required. Proper selection of filter materials and maintenance is crucial for optimal performance.
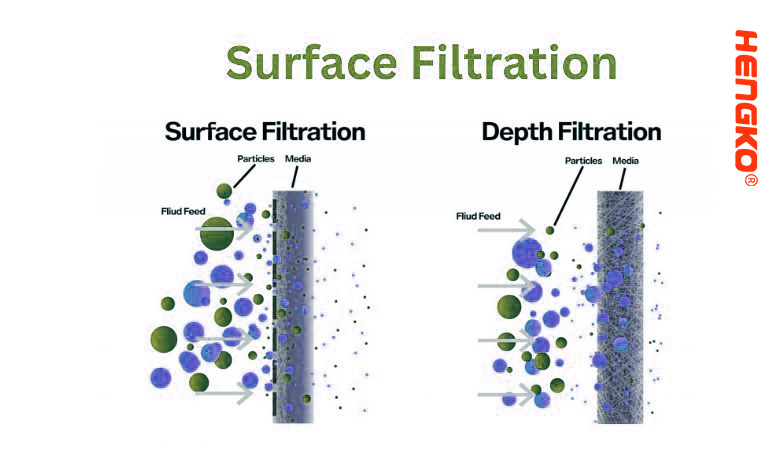
7. Surface Filtration:
Surface filtration is a method in which particles are captured on the surface of the filter medium rather than within its depth. In this type of filtration, the filter medium acts as a sieve, allowing smaller particles to pass through while retaining larger particles on its surface.
1.) Mechanism:
* Sieve Retention: Particles larger than the pore size of the filter medium are retained on the surface, much like how a sieve works.
* Adsorption: Some particles may adhere to the surface of the filter due to various forces, even if they are smaller than the pore size.
2.) Materials:
Common materials used in surface filtration include:
* Woven or non-woven fabrics
* Membranes with defined pore sizes
* Metallic screens
3.) Procedure:
* Preparation: The surface filter is positioned so that the fluid to be filtered flows over or through it.
* Filtration: As the fluid passes over the filter medium, particles are trapped on its surface.
* Cleaning/Replacement: Over time, as more particles accumulate, the filter may become clogged and need to be cleaned or replaced.
4.) Key Points:
* Defined Pore Size: Surface filters often have a more precisely defined pore size compared to depth filters, which allows for specific size-based separations.
* Blinding/Clogging: Surface filters are more prone to blinding or clogging since particles are not distributed throughout the filter but accumulate on its surface.
5.) Advantages:
* Clear Cutoff: Given the defined pore sizes, surface filters can provide a clear cutoff, making them effective for applications where size exclusion is crucial.
* Reusability: Many surface filters, especially those made from durable materials like metal, can be cleaned and reused multiple times.
* Predictability: Due to their defined pore size, surface filters offer more predictable performance in size-based separations.
6.) Limitations:
* Clogging: Surface filters can become clogged more quickly than depth filters, especially in high particulate load scenarios.
* Pressure Drop: As the filter surface becomes loaded with particles, the pressure drop across the filter can increase significantly.
* Less Tolerance to Varied Particle Sizes: Unlike depth filters, which can accommodate a broad range of particle sizes, surface filters are more selective and might not be suitable for fluids with a wide particle size distribution.
In summary, surface filtration involves the retention of particles on the surface of a filter medium. It offers precise size-based separations but is more susceptible to clogging than depth filtration. The choice between surface and depth filtration largely depends on the specific requirements of the application, the nature of the fluid being filtered, and the characteristics of the particulate load.
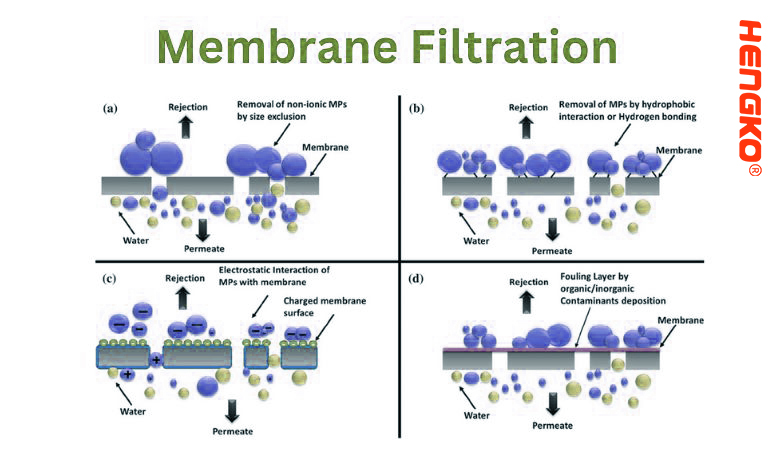
8. Membrane Filtration:
Membrane filtration is a technique that separates particles, including microorganisms and solutes, from a liquid by passing it through a semi-permeable membrane. The membranes have defined pore sizes that allow only particles smaller than these pores to pass through, effectively acting as a sieve.
1.) Mechanism:
* Size Exclusion: Particles larger than the membrane's pore size are retained on the surface, while smaller particles and solvent molecules pass through.
* Adsorption: Some particles might adhere to the membrane surface due to various forces, even if they're smaller than the pore size.
2.) Materials:
Common materials used in membrane filtration include:
* Polysulfone
* Polyethersulfone
* Polyamide
* Polypropylene
* PTFE (Polytetrafluoroethylene)
* Cellulose acetate
3.) Types:
Membrane filtration can be categorized based on pore size:
* Microfiltration (MF): Typically retains particles from about 0.1 to 10 micrometers in size. Often used for particle removal and microbial reduction.
* Ultrafiltration (UF): Retains particles from about 0.001 to 0.1 micrometers. It is commonly used for protein concentration and virus removal.
* Nanofiltration (NF): Has a pore size range that allows for the removal of small organic molecules and multivalent ions, while monovalent ions often pass through.
* Reverse Osmosis (RO): This is not strictly sieving by pore size but works based on osmotic pressure differences. It effectively blocks the passage of most solutes, allowing only water and some small solutes to pass.
4.) Procedure:
* Preparation: The membrane filter is installed in a suitable holder or module, and the system is primed.
* Filtration: The liquid is forced (often by pressure) through the membrane. Particles larger than the pore size are retained, resulting in a filtered liquid known as permeate or filtrate.
* Cleaning/Replacement: Over time, the membrane can become fouled with retained particles. Regular cleaning or replacement might be necessary, especially in industrial applications.
5.) Key Points:
* Crossflow Filtration: To prevent rapid fouling, many industrial applications use crossflow or tangential flow filtration. Here, the liquid flows parallel to the membrane surface, sweeping away retained particles.
* Sterilizing Grade Membranes: These are membranes specifically designed to remove all viable microorganisms from a liquid, ensuring its sterility.
6.) Advantages:
* Precision: Membranes with defined pore sizes offer precision in size-based separations.
* Flexibility: With various types of membrane filtration available, it's possible to target a broad range of particle sizes.
* Sterility: Certain membranes can achieve sterilizing conditions, making them valuable in pharmaceutical and biotechnological applications.
7.) Limitations:
* Fouling: Membranes can become fouled over time, leading to reduced flow rates and filtration efficiency.
* Cost: High-quality membranes and the equipment associated with them can be costly.
* Pressure: Membrane filtration often requires external pressure to drive the process, especially for tighter membranes like those used in RO.
In summary, membrane filtration is a versatile technique used for size-based separation of particles from liquids. The method's precision, coupled with the variety of membranes available, makes it invaluable for numerous applications in water treatment, biotechnology, and the food and beverage industry, among others. Proper maintenance and understanding of the underlying principles are essential for optimal results.
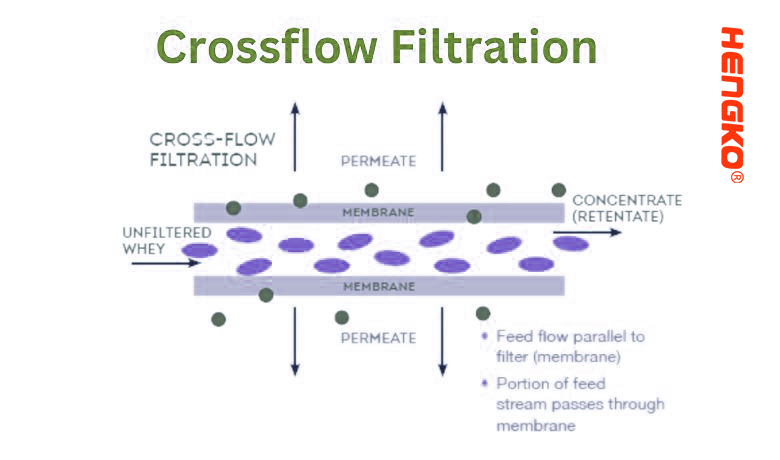
9. Crossflow Filtration (Tangential Flow Filtration):
In crossflow filtration, the feed solution flows parallel or "tangential" to the filter membrane, rather than perpendicular to it. This tangential flow reduces the build-up of particles on the membrane's surface, which is a common problem in normal (dead-end) filtration where the feed solution is pushed directly through the membrane.
1.) Mechanism:
* Particle Retention: As the feed solution flows tangentially across the membrane, particles larger than the pore size are prevented from passing through.
* Sweeping Action: The tangential flow sweeps away the retained particles from the membrane surface, minimizing fouling and concentration polarization.
2.) Procedure:
* Setup: The system is equipped with a pump that circulates the feed solution across the surface of the membrane in a continuous loop.
* Filtration: The feed solution is pumped across the membrane's surface. A portion of the liquid permeates through the membrane, leaving behind a concentrated retentate that continues circulating.
* Concentration and Diafiltration: TFF can be used to concentrate a solution by recirculating the retentate. Alternatively, a fresh buffer (diafiltration fluid) can be added to the retentate stream to dilute and wash out undesired small solutes, further purifying the retained components.
3.) Key Points:
* Reduced Fouling: The sweeping action of the tangential flow minimizes membrane fouling,
which can be a significant issue in dead-end filtration.
* Concentration Polarization:
Even though TFF reduces fouling, concentration polarization (where solutes accumulate at the membrane surface,
forming a concentration gradient) can still occur. However, the tangential flow helps in mitigating this effect to some extent.
4.) Advantages:
* Extended Membrane Life: Due to reduced fouling, membranes used in TFF often have a longer operational life compared to those used in dead-end filtration.
* High Recovery Rates: TFF allows for high recovery rates of target solutes or particles from dilute feed streams.
* Versatility: The process is suitable for a wide range of applications, from concentrating protein solutions in biopharma to water purification.
* Continuous Operation: TFF systems can be operated continuously, making them ideal for industrial-scale operations.
5.) Limitations:
* Complexity: TFF systems can be more complex than dead-end filtration systems due to the need for pumps and recirculation.
* Cost: The equipment and membranes for TFF can be more expensive than those for simpler filtration methods.
* Energy Consumption: The recirculation pumps can consume a significant amount of energy, especially in large-scale operations.
In summary, Crossflow or Tangential Flow Filtration (TFF) is a specialized filtration technique that utilizes a tangential flow to mitigate the fouling of membranes. While it offers many advantages in terms of efficiency and reduced fouling, it also requires a more intricate setup and can have higher operational costs. It's especially valuable in scenarios where standard filtration methods may rapidly lead to membrane fouling or where high recovery rates are needed.
10. Centrifugal Filtration:
Centrifugal filtration uses the principles of centrifugal force to separate particles from a liquid. In this process, a mixture is spun at high speeds, causing denser particles to migrate outwards, while the lighter fluid (or less dense particles) remains towards the center. The filtration process typically occurs within a centrifuge, which is a device designed to spin mixtures and separate them based on differences in density.
1.) Mechanism:
* Density Separation: When the centrifuge operates, denser particles or substances are forced outwards to the
perimeter of the centrifuge chamber or rotor due to the centrifugal force.
* Filter Medium: Some centrifugal filtration devices incorporate a filter medium or mesh. The centrifugal force
pushes the fluid through the filter, while particles are retained behind.
2.) Procedure:
* Loading: The sample or mixture is loaded into the centrifuge tubes or compartments.
* Centrifugation: The centrifuge is activated, and the sample spins at a predetermined speed and duration.
* Recovery: After centrifugation, the separated components are typically found in different layers or zones within the centrifuge tube. The denser sediment or pellet lies at the bottom, while the supernatant (the clear liquid above the sediment) can be easily decanted or pipetted off.
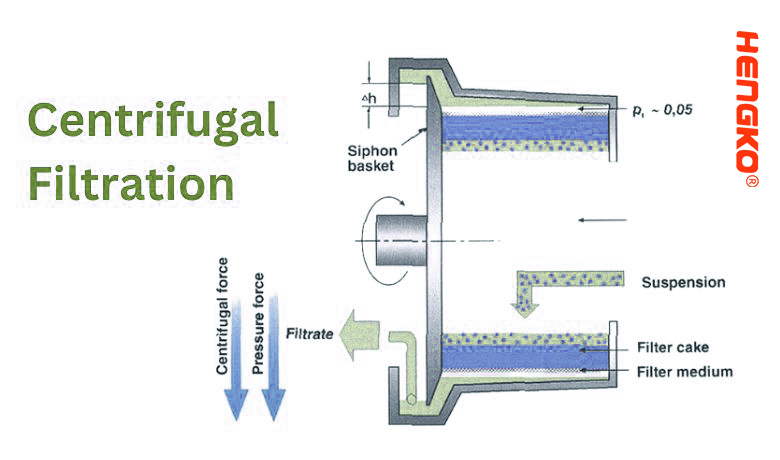
3.) Key Points:
* Rotor Types: There are different types of rotors, like fixed-angle and swinging-bucket rotors, that cater to different separation needs.
* Relative Centrifugal Force (RCF): This is a measure of the force exerted on the sample during centrifugation and is often more relevant than simply stating the revolutions per minute (RPM). RCF is dependent on the rotor radius and the speed of the centrifuge.
4.) Advantages:
* Rapid Separation: Centrifugal filtration can be much faster than gravity-based separation methods.
* Versatility: The method is suitable for a wide range of particle sizes and densities. By adjusting the centrifugation speed and time, different types of separations can be achieved.
* Scalability: Centrifuges come in various sizes, from microcentrifuges used in labs for small samples to large industrial centrifuges for bulk processing.
5.) Limitations:
* Equipment Cost: High-speed or ultra-centrifuges, especially those used for specialized tasks, can be expensive.
* Operational Care: Centrifuges need careful balancing and regular maintenance to operate safely and efficiently.
* Sample Integrity: Extremely high centrifugal forces might alter or damage sensitive biological samples.
In summary, centrifugal filtration is a powerful technique that separates substances based on their density differences under the influence of centrifugal force. It's widely used in various industries and research settings, from purifying proteins in a biotech lab to separating milk components in the dairy industry. Proper operation and understanding of the equipment are crucial to achieve the desired separation and maintain sample integrity.
11. Cake Filtration:
Cake filtration is a filtration process in which a solid "cake" or layer forms on the surface of the filter medium. This cake, which is made up of the accumulated particles from the suspension, becomes the primary filtering layer, often improving the efficiency of the separation as the process continues.
1.) Mechanism:
* Particle Accumulation: As the fluid (or suspension) is passed through the filter medium, the solid particles are trapped and start to accumulate on the filter surface.
* Cake Formation: Over time, these trapped particles form a layer or 'cake' on the filter. This cake acts as a secondary filter medium, and its porosity and structure influence the filtration rate and efficiency.
* Deepening of the Cake: As the filtration process continues, the cake thickens, which can decrease the filtration rate due to increased resistance.
2.) Procedure:
* Setup: The filter medium (could be a cloth, screen, or other porous material) is installed in a suitable holder or frame.
* Filtration: The suspension is passed over or through the filter medium. Particles begin to accumulate on the surface, forming the cake.
* Cake Removal: Once the filtration process is completed or when the cake becomes too thick, impeding the flow, the cake can be removed or scraped off, and the filtration process can restart.
3.) Key Points:
* Pressure and Rate: The filtration rate can be influenced by the pressure difference across the filter. As the cake thickens, a greater pressure difference might be needed to maintain flow.
* Compressibility: Some cakes can be compressible, which means their structure and porosity change under pressure. This can affect the filtration rate and efficiency.
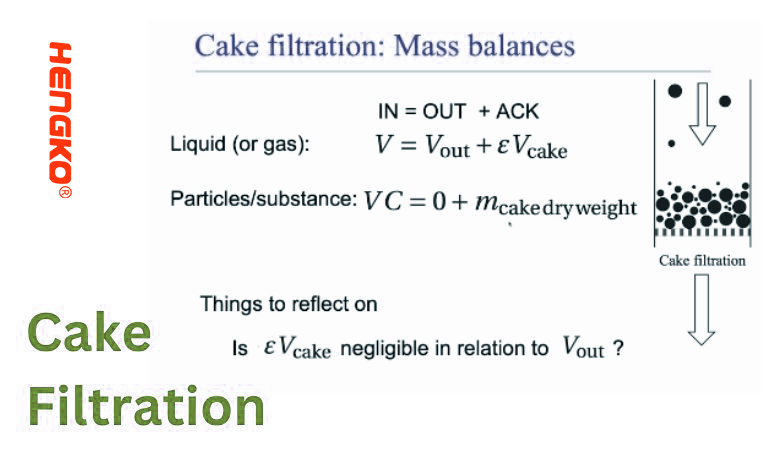
4.) Advantages:
* Improved Efficiency: The cake itself often provides finer filtration than the initial filter medium, capturing smaller particles.
* Clear Demarcation: The solid cake can often be easily separated from the filter medium, simplifying the recovery of the filtered solid.
Versatility: Cake filtration can handle a wide range of particle sizes and concentrations.
5.) Limitations:
* Flow Rate Reduction: As the cake becomes thicker, the flow rate typically reduces due to increased resistance.
* Clogging and Blinding: If the cake becomes too thick or if the particles penetrate deeply into the filter medium, it can lead to clogging or blinding of the filter.
* Frequent Cleaning: In some cases, especially with fast cake buildup, the filter may need frequent cleaning or cake removal, which can interrupt continuous processes.
In summary, cake filtration is a common filtration method in which the accumulated particles form a 'cake' that aids in the filtration process. The nature of the cake – its porosity, thickness, and compressibility – plays a crucial role in the efficiency and rate of filtration. Proper understanding and management of the cake formation are vital for optimal performance in cake filtration processes. This method is widely used in various industries, including chemical, pharmaceutical, and food processing.
12. Bag Filtration:
Bag filtration, as the name suggests, utilizes a fabric or felt bag as the filtering medium. The fluid to be filtered is directed through the bag, which captures the contaminants. Bag filters can vary in size and design, making them versatile for different applications, from small-scale operations to industrial processes.
1.) Mechanism:
* Particle Retention: The fluid flows from the inside to the outside of the bag (or in some designs, outside to inside). Particles larger than the bag's pore size are trapped within the bag, while the cleaned fluid passes through.
* Buildup: As more and more particles are captured, a layer of these particles forms on the bag's inner surface, which can, in turn, act as an additional filtration layer, capturing even finer particles.
2.) Procedure:
* Installation: The filter bag is placed inside a bag filter housing, which directs the flow of fluid through the bag.
* Filtration: As the fluid passes through the bag, contaminants are trapped inside.
* Bag Replacement: Over time, as the bag becomes loaded with particles, the pressure drop across the filter will increase, indicating the need for a bag change. Once the bag is saturated or the pressure drop is too high, the bag can be removed, discarded (or cleaned, if reusable), and replaced with a new one.
3.) Key Points:
* Material: Bags can be made from various materials such as polyester, polypropylene, nylon, and others, depending on the application and type of fluid being filtered.
* Micron Rating: Bags come in various pore sizes or micron ratings to cater to different filtration requirements.
* Configurations: Bag filters can be single or multi-bag systems, depending on the volume and rate of filtration needed.
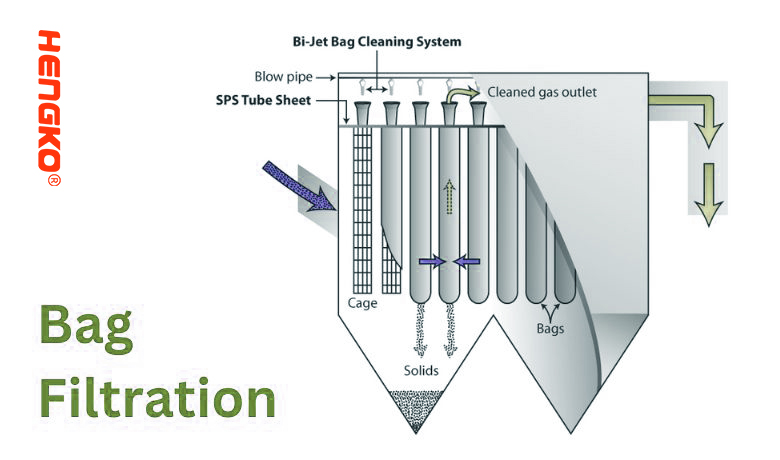
4.) Advantages:
* Cost-effective: Bag filtration systems are often less expensive than other filtration types like cartridge filters.
* Ease of Operation: Changing a filter bag is generally straightforward, making maintenance relatively easy.
* Versatility: They can be used for a wide range of applications, from water treatment to chemical processing.
* High Flow Rates: Due to their design, bag filters can handle relatively high flow rates.
5.) Limitations:
* Limited Filtration Range: While bag filters can trap a wide range of particle sizes, they might not be as effective as membrane or cartridge filters for very fine particles.
* Waste Generation: Unless the bags are reusable, spent bags can generate waste.
* Bypass Risk: If not sealed correctly, there's a chance that some fluid can bypass the bag, leading to less effective filtration.
In summary, bag filtration is a commonly used and versatile filtration method. With its ease of use and cost-effectiveness, it's a popular choice for many medium to coarse filtration requirements. Proper selection of bag material and micron rating, as well as regular maintenance, are crucial for achieving the best filtration performance.
How to Choose The Right Products of Filtration Techniques for Filtration System ?
Choosing the right filtration products is crucial for ensuring the efficiency and longevity of your filtration system. Several factors come into play, and the selection process can sometimes be intricate. Below are the steps and considerations to guide you in making an informed choice:
1. Define the Objective:
* Purpose: Determine the primary goal of filtration. Is it to protect sensitive equipment, produce a high-purity product, remove specific contaminants, or some other goal?
* Desired Purity: Understand the desired purity level of the filtrate. For instance, potable water has different purity requirements than ultra-pure water used in semiconductor manufacturing.
2. Analyze the Feed:
* Contaminant Type: Determine the nature of contaminants - are they organic, inorganic, biological, or a mixture?
* Particle Size: Measure or estimate the size of particles to be removed. This will guide the pore size or micron rating selection.
* Concentration: Understand the concentration of contaminants. High concentrations might need pre-filtration steps.
3. Consider the Operational Parameters:
* Flow Rate: Determine the desired flow rate or throughput. Some filters excel at high flow rates while others might clog quickly.
* Temperature & Pressure: Ensure the filtration product can handle the operational temperature and pressure.
* Chemical Compatibility: Ensure the filter material is compatible with the chemicals or solvents in the fluid, especially at elevated temperatures.
4. Factor in the Economic Considerations:
* Initial Cost: Consider the upfront cost of the filtration system and whether it fits within your budget.
* Operational Cost: Factor in the cost of energy, replacement filters, cleaning, and maintenance.
* Lifespan: Consider the expected lifespan of the filtration product and its components. Some materials might have a higher upfront cost but a longer operational life.
5. Evaluate Filtration Technologies:
* Filtration Mechanism: Depending on the contaminants and the desired purity, decide whether surface filtration, depth filtration, or membrane filtration is more appropriate.
* Filter Medium: Choose between options like cartridge filters, bag filters, ceramic filters, etc., based on the application and other factors.
* Reusable vs. Disposable: Decide whether a reusable or a disposable filter fits the application. Reusable filters might be more economical in the long run but require regular cleaning.
6. System Integration:
* Compatibility with Existing Systems: Ensure the filtration product can be integrated seamlessly with existing equipment or infrastructure.
* Scalability: If there's a possibility of scaling up operations in the future, choose a system that can handle increased capacity or is modular.
7. Environmental and Safety Considerations:
* Waste Generation: Consider the environmental impact of the filtration system, especially in terms of waste generation and disposal.
* Safety: Ensure the system meets safety standards, especially if hazardous chemicals are involved.
8. Vendor Reputation:
Research potential vendors or manufacturers. Consider their reputation, reviews, past performance, and after-sales support.
9. Maintenance and Support:
* Understand the maintenance requirements of the system.
* Consider the availability of replacement parts and the vendor's support for maintenance and troubleshooting.
10. Pilot Testing:
If feasible, conduct pilot tests with a smaller version of the filtration system or a trial unit from the vendor. This real-world test can provide valuable insights into the system's performance.
In summary, choosing the right filtration products requires a comprehensive evaluation of the feed characteristics, operational parameters, economic factors, and system integration considerations. Always ensure that safety and environmental concerns are addressed, and lean on pilot testing whenever possible to validate choices.
Looking for a Reliable Filtration Solution?
Your filtration project deserves the best, and HENGKO is here to deliver just that. With years of expertise and a reputation for excellence, HENGKO offers tailored filtration solutions to meet your unique requirements.
Why Choose HENGKO?
* Cutting-edge technology
* Customized solutions for diverse applications
* Trusted by industry leaders worldwide
* Committed to sustainability and efficiency
* Don't compromise on quality. Let HENGKO be the solution to your filtration challenges.
Contact HENGKO Today!
Ensure the success of your filtration project. Tap into HENGKO's expertise now!
[ Click As Follow to Contact HENGKO]
Send your message to us:
Post time: Aug-25-2023